I was just about to use a skincare product sent by a brand when I received a call from their customer service team informing me that the batch was faulty. That’s unsettling to say the least.
I’d be hesitant to shop with them again. Once a mistake like that happens, it’s hard to take the risk again. This is where the best quality management systems (QMS) become crucial for businesses.
A well-implemented QMS helps businesses catch issues early, ensuring product quality and compliance with industry standards. By streamlining processes, tracking production quality, and providing visibility into every stage of the product lifecycle, QMS minimizes the risk of defects making it to the market.
For any business, a reliable QMS isn’t just a good practice—it’s essential for maintaining customer trust and product integrity. If you’re serious about ensuring top-notch quality control, investing in the right QMS software is the way forward.
9 best quality management software: my picks for 2025
-
MasterControl: Best for end-to-end QMS in regulated life sciences
for quality and document management, with built-in compliance.
(pricing available on request)
-
Qualio: Best for small to mid-sized life sciences companies for compliance
For growing life sciences companies, we offer tools for documentation.
(pricing available on request)
-
Ideagen Quality Management is best for industry-specific compliance and risk. It is also best for audit management, compliance tracking, and risk mitigation.
(pricing available on request)
-
ETQ Reliance QMS: Best for flexible automation and analytics
To automate quality processes, apply analytics, and maintain compliance. (pricing available on request)
-
Greenlight Guru Quality Management System: Best for medical device product lifecycle
For medical device companies, combining QMS and product development tools. (pricing available on request)
-
SafetyCulture: Best for operational safety and inspection-driven quality
For safety and quality through inspection checklists, mobile audits, and real-time reporting. (free plan available, pricing for premium plan starts at $24/seat/mo)
-
QT9 QMS: Best for modular cloud-based QMS with CAPA
For document management, CAPA, audits, training, and supplier management. (free trial available, pricing for plans available on request)
-
AmpleLogic eQMS: Best for customizable quality control in Manufacturing
For streamlining quality processes and operational efficiency in manufacturing.(pricing available on request)
-
ZenQMS: Best for user-friendly QMS in life sciences and pharma
Best for compliance-heavy industries, offering intuitive tools for managing, training, audits, and document control. (pricing available on request)
* These quality management tools are top-rated in their category, according to G2 Grid Reports.
My top 9 quality management software recommendations for 2025
The best quality management software (QMS) is designed to help organizations ensure product quality, maintain compliance, and improve operational efficiency. As I evaluated these tools, I saw firsthand how they simplify document control, automate work, and enhance collaboration across teams, ensuring consistent and high-quality output.
Companies that implement quality management experience an average of 9% increase in sales, and 26% increase in profitability, according to a survey by gosteelhead, which indicates the positive outcome of software adoption.
How did I find and evaluate the best quality management software?
By analyzing G2 reviews and G2’s Grid Reports, I gained a comprehensive understanding of each quality management software's features, ease of use, and overall value. I relied on verified reviews from industry professionals with hands-on experience to validate my findings.
Additionally, I used AI to analyze patterns in user feedback, ensuring I captured diverse perspectives on the software’s effectiveness in streamlining quality control processes, improving collaboration, and maintaining compliance. The screenshots featured in this article may be a mix of those from G2 reviews and vendor-provided visuals.
By combining G2 insights with expert feedback, I’ve compiled a list of the best quality management software to help you choose the right tool for your organization’s needs.
What makes quality management software worth it: My opinion
According to Grand View Research, the global quality management software market size was estimated at USD 11.14 billion in 2024 and is projected to reach USD 20.66 billion by 2030, at a CAGR of 10.6% from 2024 to 2030.
When evaluating quality management software (QMS), I focus on several key features to decide its effectiveness for streamlining operations and ensuring compliance:
- Audit trails and traceability: One of the core strengths of a QMS is the ability to track and trace every change made to a document or process. I evaluate the system’s ability to create an audit trail, which records each modification, who made it, and when it occurred. This feature ensures compliance with industry standards, offering full transparency for regulatory audits and internal reviews.
- Process management and workflow automation: Quality management software should streamline processes by automating workflows, approvals, and notifications. I assess how the system supports the design, tracking, and management of business processes, such as non-conformance management or corrective action requests. The ability to automate routine tasks, such as notifying teams of required actions or approval, saves time and reduces the chance for error.
- Real-time reporting and analytics: For quality management to be effective, data should be captured and analyzed in real time. I look for systems that allow for easy generation of reports related to quality control, audit results, and performance metrics. The software should also include analytics tools to help identify patterns, bottlenecks, or areas of improvement, enabling teams to make data-driven decisions that improve quality over time.
- Compliance management and standardization: A key aspect of QMS is ensuring that all processes comply with industry standards and regulations. I evaluate the system's ability to manage certifications, audit schedules, and compliance tracking. The software should also support standardization of procedures, making it easy to maintain consistency across operations and ensure that all team members follow the same protocols.
- Risk management and corrective actions: A robust QMS should help identify, track, and mitigate risks in operations. I evaluate how well the software supports risk management by allowing teams to log potential issues, assess their severity, and use corrective actions. The system should automate the process of escalating critical issues, assigning responsibilities, and tracking resolutions to ensure that risks are managed proactively and corrective actions are taken in a timely manner.
- Support engineering change management for quality improvement
- Monitor nonconformance and out-of-specification products and components
- Provide a database of risk and compliance information and documentation
- Generate documents for incidents and quality control activities
- Provide training and certification to comply with quality standards and regulations
- Ensure quality and compliance for the fixed assets used by the company
- Include functionality to monitor supplier quality and performance
- Assist with corrective actions and preventive actions (CAPA) for quality issues
- Deliver customizable workflows and checklists for inspections and audits
This data was pulled from G2 in 2025. Some reviews have been edited for clarity.
1. MasterControl: Best for end-to-end QMS in regulated life sciences
MasterControl is widely recognized as a robust platform, particularly for businesses in the life sciences industry.
Based on 354 G2 reviews, MasterControl has a customer satisfaction score of 89, and 87% of users are willing to recommend it to others.
One feature that I see getting a lot of praise is its system validation, which is especially valuable in regulated industries. G2 users often highlight how MasterControl meets the strictest regulatory standards, with some pointing out its adoption by departments within the FDA. This validation is consistently noted as a key selling point for its credibility and reliability, making it a trusted choice for managing compliance in regulated industries.
Another aspect frequently appreciated by G2 reviewers is the platform's integrated nature. According to feedback I gathered from G2 users, the system’s ability to centralize document management, training, audits, and quality events is highly valued. Users often emphasize how this integration eliminates the need for scattered paper files or multiple systems, making their workflow more efficient and reducing errors. This all-in-one system is particularly praised for streamlining operations and improving overall productivity.
One standout capability, according to users, is document management. G2 reviewers often mention how the system has simplified document storage, tracking, and access. This feature is seen as a major advantage, particularly for companies that were previously managing physical or disorganized digital files. Many users report that MasterControl significantly improves organization and productivity, contributing to a smoother operational flow.
Based on my review of G2 user feedback, the electronic signature functionality is another widely praised feature. For businesses with multiple locations or remote teams, this capability is essential, and users frequently highlight how MasterControl facilitates seamless collection of electronic signatures across different regions. Many G2 users from the US, Canada, and the UK appreciate how this feature saves time and ensures compliance with international standards.

According to G2 user reviews, MasterControl is respected for its compliance strength, though some businesses outside life sciences find it overly complex. Detailed process modules, such as multi-step CAPA management, are powerful for regulated industries but may feel excessive for organizations with lighter quality management needs.
Another recurring theme in G2 feedback relates to licensing structure. Reviewers mention that the distinction between “basic” and “full” licenses can complicate collaboration, since only full license holders are able to approve or edit documents, creating friction in workflow management.
Overall, G2 sentiment reflects MasterControl as a trusted platform for regulated industries, delivering strong compliance and process control even if licensing and complexity pose challenges for broader use cases.
What I like about MasterControl:
- I’ve seen G2 reviewers consistently praise MasterControl for its robust system validation, particularly its ability to meet strict regulatory standards in the life sciences industry.
- From my review of user feedback, the integrated platform stands out, with many users highlighting how it centralizes document management, training, audits, and quality events for improved workflow efficiency.
What G2 users like about MasterControl:
"All of the different portals are quite similar in how they are used, and therefore what you learn to do or establish in one often translates to another. So learning it and gaining competency is fairly quick. As a business involved in the life sciences, we rely on it for use during audits with the governing body."
- MasterControl Review, Deona L.
What I dislike about MasterControl:
- I’ve noticed that non-life sciences businesses often find MasterControl's complexity a barrier, with users noting its detailed process modules as cumbersome for companies with fewer regulatory requirements.
- Multiple reviewers have expressed dissatisfaction with MasterControl’s licensing structure, pointing out that the distinction between "basic" and "full" licenses creates workflow friction and complicates user management.
What G2 users dislike about MasterControl:
"Most of the software is centered around life sciences and the medical industry. This leads to the inclusion of many more checks and balances in the system than we require as a manufacturer that only seeks to meet to ISO 9001. There are configuration options available to fit our situation, so this is only a minor issue."
- MasterControl Review, Travis B.
Strengthen your organization's resilience with top-rated GRC software today.
2. Qualio: Best for small to mid-sized life sciences companies for compliance
Qualio is widely recognized as a solid tool for streamlining document management and approval processes.
Based on 553 G2 reviews, Qualio has got a 100 satisfaction score and over 88% users say that they are likely to recommend it to others in the overall business segment.
One feature that G2 reviewers frequently highlight is the user interface, which is praised for being clean and intuitive. Many users mention how easy it is to navigate the platform, even for team members with minimal technical expertise. G2 feedback shows that the simplicity and clarity of the layout significantly improve the user experience, helping teams get up to speed quickly and use the platform efficiently.
Another element that G2 reviewers often appreciate is the review and approval functionality. Many users note how efficient and straightforward the process is for organizing and approving documents. This feature seems to stand out, with several reviewers mentioning that it significantly reduces complexity in document handling, making the overall process smoother and more manageable for teams.
I’ve noticed that document editing and collaboration within Qualio is another strong point in G2 reviews. G2 users consistently highlight how user-friendly this feature is, with many pointing out the ease of leaving comments and suggestions during the editing process. This feature is widely regarded as effective for improving document revisions, especially in complex projects where multiple feedback loops are common. The ability to manage change controls and create form templates is also frequently praised for saving time and streamlining documentation tasks.
Looking at G2 feedback, Qualio’s integration with the Microsoft Suite is mentioned as a notable benefit. Users frequently comment on how easy it is to import documents from tools like Microsoft Word, with some mentioning minor formatting discrepancies. Despite these small issues, most G2 reviewers appreciate the seamless transition from familiar tools, making it easier to incorporate documents into Qualio without significant hassle.

According to G2 user reviews, Qualio is appreciated for streamlining quality management, though the review and approval process could be smoother. Some users mention frustration with the inability to accept edits without affecting reviewer sign-off timestamps, which complicates audit trails that are critical for compliance.
Another theme in G2 feedback relates to document handling. Reviewers note that formatting options are limited compared to tools like Word or Google Docs, and a few also mention that documents can freeze during heavy collaboration, slowing real-time reviews.
Overall, G2 sentiment reflects Qualio as a valuable platform for regulated teams, providing strong compliance and quality management, even if formatting and review workflows leave room for refinement.
What I like about Qualio:
- I’ve seen G2 reviewers frequently praise Qualio's user interface for being clean and intuitive, making it easy for non-technical team members to navigate and use it efficiently.
- From my review of user feedback, document editing and collaboration stand out as strong features. Many users appreciate how easy it is to leave comments and manage change controls during revisions.
What G2 users like about Qualio:
"The search & tags features are excellent for quickly finding documents, allowing me to put the necessary paperwork for production together in a timely manner without spending ages hunting for specific documents in hidden folders like our old OneDrive system."
- Qualio Review, Rob C.
What I dislike about Qualio:
- I’ve noticed frustration from users regarding the review and approval process, particularly the inability to accept edits without losing reviewer sign-off timestamps, which affects compliance and the audit trail.
- Multiple G2 reviewers have expressed dissatisfaction with document formatting, pointing out that the platform lacks flexibility compared to tools like Microsoft Word, leading to formatting issues.
What G2 users dislike about Qualio:
"The reporting capabilities are somewhat limited—you can get the basics, but for more complex metrics, you may find yourself working offline. Also, the change control workflows could benefit from a bit more flexibility for organizations with more customized approval processes. Lastly, while onboarding is generally smooth, the initial configuration requires a clear understanding of how your quality system maps into Qualio’s structure—otherwise, you may end up reorganizing later."
- Qualio Review, Verified User in Medical Devices
3. Ideagen Quality Management: Best for industry-specific compliance and risk
After reviewing G2 user feedback on Ideagen Quality Management, I’ve found that it’s generally well-regarded for its ability to streamline quality management processes, particularly for maintaining ISO 9001 compliance.
Based on 405 G2 reviews, Ideagen has received a customer satisfaction score of 75, with over 88% users willing to recommend it to others in the overall business segment.
A consistent theme in G2 reviews is how the platform simplifies tracking compliance across various departments, which saves time and ensures that operations run smoothly. Many users have expressed appreciation for the tool’s ability to help businesses stay aligned with international quality standards, a crucial feature for maintaining industry credibility.
One feature that I frequently see G2 reviewers highlight is the audit management capabilities. Users often praise how Ideagen Quality Management facilitates scheduling and managing audits, making it easier to maintain compliance and efficiency.
The integration of operational risk management tools is another standout aspect, with many reviewers noting how it helps identify potential issues before they escalate. This proactive approach seems to be a key factor in preventing operational disruptions, according to feedback from multiple users.
Another element that G2 users often call out is the tool's focus on uniformity across teams. The structured workflows and automated approvals ensure that all teams follow the same procedures, a feature that reviewers particularly appreciate for maintaining consistency and quality. From the feedback I gathered, employees value how clear and straightforward the platform's processes are, which directly contributes to better team performance and efficiency.

According to G2 user reviews, Ideagen Quality Management is valued for its comprehensive feature set, though new users often face a steep learning curve. The breadth of functionality can feel overwhelming at first, and onboarding may take longer than expected for teams needing quick adoption.
Another recurring theme in G2 feedback is around reporting. While the built-in reports provide a solid starting point, several reviewers note that significant customization is needed to meet specific business requirements, which can be time-consuming for those unfamiliar with the process.
Overall, G2 sentiment reflects Ideagen Quality Management as a robust compliance and quality platform, with strong long-term value once users move past the initial learning curve and customization effort.
What I like about Ideagen Quality Management:
- I’ve noticed G2 reviewers frequently praise audit management capabilities, particularly for their ability to streamline scheduling and compliance tracking, making audits more efficient.
- From my review of user feedback, risk management integration stands out as a key feature, with users appreciating how it helps identify potential issues before they escalate, preventing operational disruptions
What G2 users like about Ideagen Quality Management:
"The Ideagen Quality Management System is an all-in-one solution to our quality management needs and is vital to our laboratory practices. It allows for all our quality and governance work to be performed and recorded in one place and has been in use within our pathology laboratory for over 10 years. Accreditation evidence is easy to gather and is well-liked by our independent assessment body. The system is easy and intuitive to use by all members of staff on a daily basis. Without it, our quality and governance systems would be much more complex. It is a system we could not function without."
- Ideagen Quality Management Review, Wallis H.
What I dislike about Ideagen Quality Management:
- I’ve seen some users express frustration with the learning curve, particularly due to the platform’s broad range of features, which can be overwhelming for new users during onboarding.
- Based on my review of G2 feedback, reporting capabilities evoke mixed reactions. Some users mention the need for significant customization to meet specific business needs, which can be time-consuming.
What G2 users dislike about Ideagen Quality Management:
"The back-end management/admin can be cumbersome and limited in some areas. Additionally, extracting data can be an issue, as we sometimes have to copy data to extract it, which includes unnecessary data."
- Ideagen Quality Management Review, Alex F.
Explore top ERP systems that integrate quality management seamlessly across your operations.
4. ETQ Reliance QMS: Best for flexible automation and analytics
After reviewing G2 user feedback on ETQ Reliance QMS, I’ve noticed that the platform is generally praised for its mobile accessibility.
Based on 539 reviews, ETQ Reliance has received a customer satisfaction score of 83. Users are likely to recommend it to others at a rate of 88% in the overall business segment.
Many G2 users highlight how the ability to access the system while on the go has been a major advantage for their teams. This feature is often mentioned as a productivity booster, as it allows staff to stay connected and engaged with critical processes even when they’re not at their desks. I’ve seen multiple reviewers express how important this flexibility is in today’s fast-paced, mobile-driven work environment.
Another feature frequently mentioned in G2 reviews is the community access through ETQ Campus. Users appreciate the live videos and webinars that allow them to deepen their understanding of the platform and stay up to date with new features and best practices. A recurring theme in the feedback is the value of the community for troubleshooting and expanding users' knowledge, which is seen as a great resource for ongoing learning. Many reviewers mention how helpful the platform's community-driven content has been in resolving issues and improving their overall experience.
Customer support is another area that G2 reviewers consistently give high marks. ETQ’s customer support team is often described as knowledgeable and responsive, making it easier for users to get assistance when needed. A common piece of feedback I’ve seen is that the support team’s willingness to work with users to address questions or problems significantly contributes to a smoother experience. This level of service seems to instill confidence in users, knowing that help is readily available.

According to G2 user reviews, ETQ Reliance QMS is praised for its flexibility, though some users wish for better documentation and indexing. Several reviewers mention that the absence of a general overview document or index tied to the search bar makes setup and configuration more time-consuming than it should be.
Another theme in G2 feedback relates to customization and the learning curve. While the platform is highly adaptable, exploring enhanced functionality often requires a patient, step-by-step approach, and users note that not all features are as clearly documented as they would like.
Overall, G2 sentiment reflects ETQ Reliance QMS as a powerful and customizable platform that delivers strong long-term value once teams work through the initial setup and learning curve.
What I like about ETQ Reliance QMS:
- I’ve noticed that many G2 reviewers appreciate ETQ Reliance QMS's mobile accessibility, highlighting how it boosts productivity by keeping teams connected while on the go.
- From what I’ve seen, users frequently praise the community access through ETQ Campus for offering valuable live videos and webinars that help them stay informed and solve issues.
What G2 users like about ETQ Reliance QMS:
"One of the things I love about ETQ Reliance QMS is how easy it makes everything. It’s super customizable, allowing you to adjust it to fit exactly what you need, which saves a lot of hassle. Everything’s consolidated in one place—document control, CAPA, and more—so there’s no need to jump between systems. Additionally, its user-friendly interface ensures that the whole team can adapt quickly without feeling overwhelmed. It truly simplifies managing quality."
- ETQ Reliance QMS Review, Komal M.
What I dislike about ETQ Reliance QMS:
- I’ve read multiple reviews where users express frustration about the lack of a general overview document or index, which could streamline the initial setup and feature configuration.
- I’ve come across feedback mentioning the challenge of exploring advanced functionality, with users noting that the customization process often requires patience and more detailed documentation.
What G2 users dislike about ETQ Reliance QMS:
"Though many REST API features have been added, the documentation on API usage needs improvement. Some key features, such as the ability for users to save view filters and additional controls in view configuration and centralized reports, are still missing."
- ETQ Reliance QMS Review, Sangamesh P.
5. Greenlight Guru Quality Management System: Best medical device product lifecycle
Greenlight Guru Quality Management System is widely praised for its comprehensive coverage of quality management functions.
Based on 329 G2 reviews, Greenlight has received a customer satisfaction score of 59, with over 91% of users likely to recommend it to others in the overall business segment.
One feature that G2 users consistently highlight is the system's ability to handle a broad range of quality management processes. From document and change control to design controls, risk management, and continuous monitoring and improvement, many reviewers appreciate having a single platform to manage all these tasks. This integration is particularly valuable in the medical device industry, where compliance and risk management are essential. Many G2 reviewers emphasize how this centralized approach simplifies workflows and boosts efficiency.
Another point of praise I’ve seen from G2 reviewers is the user-friendly interface. Based on user feedback, many find the platform easy to learn, which makes transitions smoother for teams adopting the system. I’ve read multiple G2 reviews where users mention that the simple interface helped them quickly navigate the platform and adapt to its features without a steep learning curve. This is often highlighted as a major benefit for teams that need to get up to speed quickly.
Automation is another feature G2 users frequently appreciate. Many users mention how Greenlight Guru allows them to customize the system according to their specific needs, which significantly reduces manual work. This automation feature has been particularly helpful for streamlining routine tasks, ensuring consistency, and improving accuracy. According to feedback, it’s considered a major time-saver by those who rely on the system for quality management processes.
The integration of pre-configured validation packages is another standout capability, as highlighted by G2 reviewers. Many users mention that these packages made it much easier to meet their QMS requirements without needing to spend time customizing from scratch. This is praised as a major convenience, particularly for teams looking to avoid unnecessary delays in their operations. Additionally, the module training videos received positive feedback for helping users quickly understand how to use the system effectively, allowing employees to start using the platform with confidence.

According to G2 user reviews, Greenlight Guru QMS is valued for its focus on medical device compliance, though onboarding can be a hurdle. Some reviewers mention that uploading legacy documents—particularly in batches—feels cumbersome, and they would prefer a smoother bulk upload option during setup.
Another theme in G2 feedback relates to workspace consistency. Users note that certain fields require manual saving while others don’t, and differences in input formats across workspaces (such as RTF text boxes in some areas but not others) create confusion and slow down workflows.
Overall, G2 sentiment reflects Greenlight Guru QMS as a compliance-driven and reliable quality management tool, with most drawbacks tied to onboarding efficiency and workspace uniformity.
What I like about Greenlight Guru Quality Management System:
- I’ve noticed that many G2 users praise the comprehensive coverage of quality management functions in Greenlight Guru, with many highlighting how it integrates document control, risk management, and compliance processes.
- From what I’ve seen, reviewers often appreciate the user-friendly interface, which allows teams to quickly adapt to the platform without a steep learning curve, making transitions smoother.
What G2 users like about Greenlight Guru Quality Management System:
"Our company transitioned to Greenlight Guru over two years ago after previously being fully paper-based. Greenlight Guru has simplified document control and change order processes, making them easy to follow. The recent updates to automate the Training Workspace have been effective and seamlessly integrated into our processes. Additionally, the Greenlight Guru team is always responsive and helpful with both software and industry-related questions."
- Greenlight Guru Quality Management System Review, Verified User in Medical Devices.
What I dislike about Greenlight Guru Quality Management System:
- I’ve come across feedback where users express frustration with the cumbersome process of uploading legacy documents in batches, wishing for a more efficient mass data upload option.
- I’ve seen multiple users mention issues with inconsistency across workspaces, particularly the manual saving of field entries and varying data input formats, which creates confusion and hinders workflows.
What G2 users dislike about Greenlight Guru Quality Management System:
"The initial setup or migration to Greenlight Guru can feel cumbersome. Also, the email notifications can’t be sorted, leading to a flood of updates from all departments, even for items you're not involved with."
- Greenlight Guru Quality Management System Review, Sharath M.
6. SafetyCulture: Best for operational safety and inspiration-driven quality
After evaluating SafetyCulture based on user feedback from G2, I’ve noticed that many reviewers are highly satisfied with the tool's ease of use.
Based on 37+ verified G2 reviews, SafetyCulture has achieved a customer satisfaction score of 65. Over 92% of users are willing to recommend it to others for its mobile access, flexibility, and workflows.
One consistent theme in the reviews is how intuitive the layout is, making it easy to navigate without requiring extensive training. G2 users frequently highlight how this ease of use is particularly beneficial for managing large amounts of equipment. For example, some users, like those managing thousands of pieces of equipment daily, report that SafetyCulture significantly streamlines their workflow, saving both time and effort.
Another feature that G2 reviewers frequently appreciate is the software's organization. Based on the feedback I’ve reviewed, users seem to value how easy it is to upload data into vital reports. Many reviewers mention how quickly they can input necessary information and generate reports without getting bogged down by complicated steps. This simple and efficient process appears to make data handling less stressful and allows teams to stay accurate while focusing on other important tasks.
From what I’ve gathered from G2 feedback, SafetyCulture’s flexibility is another aspect that stands out. Reviewers often mention how the software can handle a variety of equipment types, from newer models to older ones, without feeling restrictive. Users appreciate how the platform accommodates different needs, from tracking maintenance schedules to ensuring compliance, and everything seems well-organized and easily accessible.

According to G2 user reviews, SafetyCulture is praised for its inspection and audit capabilities, though some users report challenges with AI accuracy. A few reviewers note that while the AI assists with report generation, occasional errors or misinterpretations can slow down final outputs.
Another theme in G2 feedback relates to accessing historical data. Users mention that recalling older inspections isn’t always intuitive, as navigation requires multiple steps, and they would prefer a more streamlined way to retrieve past records quickly.
Overall, G2 sentiment reflects SafetyCulture as a powerful tool for inspections and workplace safety, with most drawbacks tied to AI accuracy and historical data navigation.
What I like about SafetyCulture:
- I’ve noticed many G2 users appreciate the tool's user-friendliness. Its intuitive layout makes it easy to navigate and saves time, especially for teams managing large amounts of equipment.
- From the feedback I’ve reviewed, data organization is frequently praised for making it simple to upload and generate reports, allowing teams to stay accurate while managing their workflows efficiently.
What G2 users like about SafetyCulture:
"This software is very user-friendly and boosts our daily efficiency by helping us track thousands of pieces of equipment. Its organization, easy data uploads for vital reports, and wide range of features make it more convenient than similar tools I've used at other facilities."
- SafetyCulture Review, Daniel D.
What I dislike about SafetyCulture:
- I’ve seen several G2 users express frustration with AI errors when generating reports, noting occasional delays or inaccuracies in work area analysis that affect the tool's reliability.
- I’ve encountered feedback mentioning difficulty accessing historical data, with users suggesting that a more intuitive method for retrieving past inspections could improve the overall experience.
What G2 users dislike about SafetyCulture:
"SafetyCulture is effective for high-level task tracking, but it could improve by offering more detailed feedback on completed inspections and making it easier to navigate and retrieve past inspections across multiple screens."
- SafetyCulture Review, Dona B.
7. QT9 QMS: Best for modular cloud-based QMS with CAPA
Based on my review of G2 feedback, it’s clear that many users appreciate the positive impact QT9 QMS has had on their quality management processes.
Based on 103 verified G2 reviews, QT9 has received a customer satisfaction score of 84, with over 97% people willing to recommend it to others in the overall business segment.
A consistent theme I’ve noticed is how the system streamlines operations, making processes more efficient and ensuring that companies always comply with industry standards. Several G2 reviewers highlight the peace of mind that comes with having a validated system in place, which seems to be one of the major selling points for users who value precision and compliance.
Another feature that stands out in G2 reviews is the automation of approval workflows. Many users, like myself, appreciate how this feature saves time and ensures consistency across the organization. This automation is frequently mentioned as a game-changer for improving efficiency, reducing human error, and maintaining regulatory compliance. Reviewers also note how the system makes it easier to track compliance in real-time, ensuring nothing is missed in the process.
G2 users also tend to praise the inclusion of electronic signature approvals and automated reminders. Based on the feedback I reviewed, these features are highly valued for their ability to ensure timely approvals and follow-ups. Multiple reviewers highlight how automation helps keep everything on track, making it easier to meet regulatory standards and stay compliant without manual oversight.
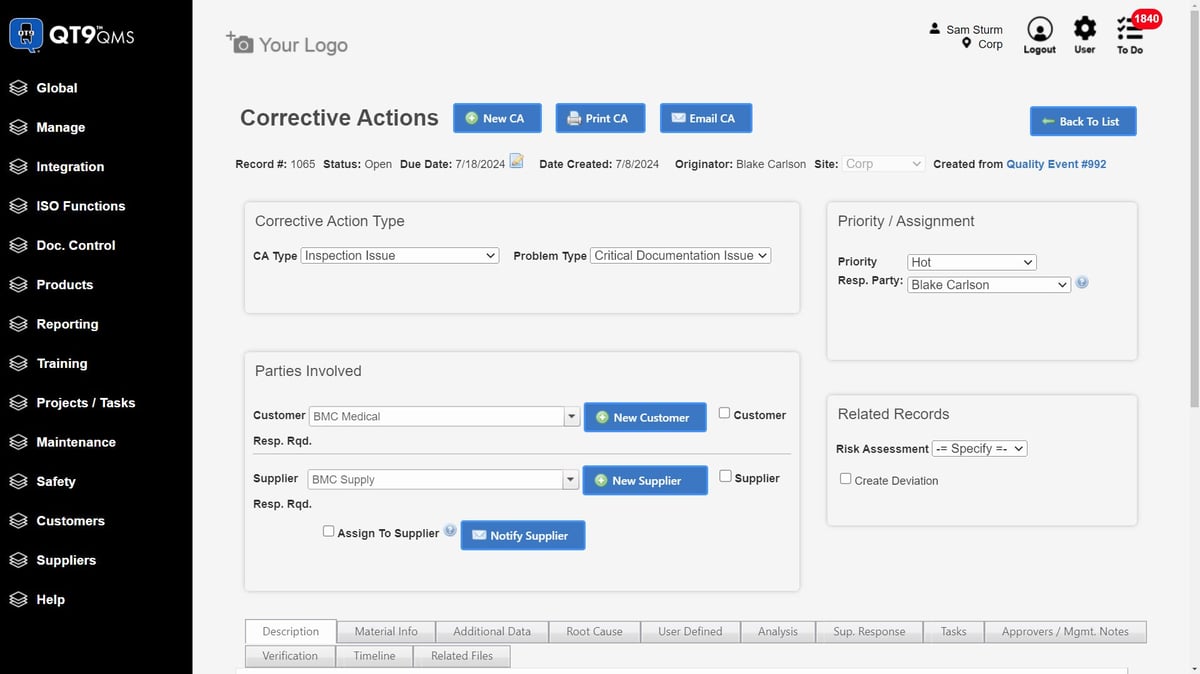
According to G2 user reviews, QT9 QMS is valued for its ease of use and compliance support, though some users note gaps in the customer document portal. Specifically, notifications for document revisions only go to internal staff, requiring teams to manually alert customers—a step many feel could be automated for efficiency.
Another theme in G2 feedback relates to dashboard customization. While the functionality works well, reviewers mention that more flexibility in tailoring the layout would make it easier to adapt to individual workflows.
Overall, G2 sentiment reflects QT9 QMS as a reliable and user-friendly quality management system, with most drawbacks tied to customer notifications and dashboard personalization.
What I like about QT9 QMS:
- I’ve noticed that many G2 users appreciate how automation of approval workflows improves efficiency, reduces human error, and ensures compliance across organizations.
- From the feedback I’ve reviewed, electronic signature approvals and automated reminders are highly valued for ensuring timely approvals and follow-ups, helping users stay compliant without manual oversight.
What G2 users like about QT9 QMS:
"From the start of our quality journey to find the right QMS for our org, it was clear that QT9 was the right fit. The support and demonstrations leading up to the point of integration were in another category compared to other potential providers that we had meetings with. The ability to automate approval workflows, track regulatory compliance, and manage multiple sites from one dashboard is a game-changer. Plus, the electronic signature approvals and automated reminders ensure everything stays on track and within compliance at all times. QT9 has made our quality management processes more streamlined and efficient, and the fact that it’s fully validated gives us peace of mind."
- QT9 QMS Review, Jason C.
What I dislike about QT9 QMS:
- I’ve encountered dissatisfaction regarding the Customer Document Portal. Users note that it lacks automatic notifications for customers, leading to inefficiencies in communication.
- I’ve seen several G2 users mention a need for more dashboard customization options, as greater flexibility in personalizing the dashboard would enhance usability for many users.
What G2 users dislike about QT9 QMS:
"The only downside I've experienced at this point is the lack of integration into our ERP. It's a standalone system that does have open API's, but it would be nice if it were compatible out of the box."
- QT9 QMS Review, Joshua E.
8. AmpleLogic Electronic eQMS: Best for customizable quality control in manufacturing
From my review of G2 feedback, it’s clear that many users have a positive view of AmpleLogic Electronic Quality Management System (eQMS), particularly for its user-friendly interface.
Based on 56 verified G2 reviews, AmpleLogic has received a customer satisfaction score of 81, and 98% of users are willing to recommend it to others in the overall business segment.
Multiple reviewers mention how easy the system is to navigate, even for new users. This simplicity allows for quick adaptation without the need for extensive training, which has been highlighted as a major benefit in streamlining quality and regulatory compliance efforts. G2 users often emphasize how intuitive the design makes it easy to keep operations running smoothly without heavy reliance on technical support.
Another feature that consistently stands out in user feedback is the graphical presentations and system-generated reports. Several G2 reviewers have praised this feature for helping them easily interpret data and trends, especially when managing complex quality tasks. Many users have highlighted the time-saving aspect of being able to automatically generate reports that align with regulatory standards, making it easier to present findings to stakeholders and maintain consistency in documentation.

According to G2 user reviews, AmpleLogic is appreciated for its low-code configurability, though some users point to gaps in system investigation and risk assessment features. Without these built-in, teams often rely on external tools, which adds extra steps and reduces overall efficiency in critical quality tasks.
Another theme in G2 feedback relates to audit trail reporting. While the system tracks actions and changes, reviewers mention that the reports could be more comprehensive and user-friendly, noting that stronger implementation would further enhance compliance and regulatory readiness.
Overall, G2 sentiment reflects AmpleLogic as a flexible and adaptable QMS platform, with opportunities to strengthen risk management and audit reporting features for even greater impact.
What I like about AmpleLogic Electronic Quality Management System (eQMS):
- I’ve noticed that many G2 users appreciate the user-friendly interface of AmpleLogic eQMS, which allows for quick adaptation and smooth operations without the need for extensive training.
- From the feedback I’ve reviewed, graphical presentations and system-generated reports stand out as time-saving features that help users interpret data and meet regulatory standards efficiently.
What G2 users like about AmpleLogic Electronic Quality Management System (eQMS):
"Amplelogic QMS system has all the required quality-related modules, and all the deviations, change controls, and CAPAs are effectively maintained and tracked, and all the tools used for the investigations are good. This system is aligned with our workflow, and the users can access it very easily as it has a user-friendly interface and can be easily integrated with other applications."
- AmpleLogic Electronic Quality Management System (eQMS) Review, Kandikattu K.
What I dislike about AmpleLogic Electronic Quality Management System (eQMS):
- I’ve come across feedback highlighting the absence of system investigation and risk assessment features, which forces users to rely on external tools, creating inefficiencies and added complexity.
- I’ve seen some G2 reviewers mention dissatisfaction with the Audit Trail Reports, noting that improvements to these reports could enhance compliance capabilities and provide a more comprehensive history of actions.
What G2 users dislike about AmpleLogic Electronic Quality Management System (eQMS):
"The software lacks a draft-saving feature, preventing users from saving partially completed prepaid entries."
- AmpleLogic Electronic Quality Management System (eQMS) Review, Manilal P.
9. ZenQMS: Best for user-friendly QMS in life sciences and pharma
Based on my review of G2 user feedback, ZenQMS generally receives positive evaluations, especially for its ease of use and customer support.
Based on 91 verified G2 reviews, ZenQMS has received a customer satisfaction score of 58, with 94% users willing to recommend it to others in the overall business segment.
A consistently highlighted feature by G2 users is the intuitive nature of the platform. Many reviewers mention how simple it is to get up to speed with ZenQMS, which doesn’t require extensive training. I’ve seen users particularly appreciate the short and effective training modules, which helped them understand the key functionalities of the software quickly. This ease of learning and smooth onboarding process appears to be a major benefit for users, as several reviewers noted that they were able to get started without much hassle.
Another aspect that stands out in G2 reviews is the smooth implementation process. From the feedback I gathered, users often praise ZenQMS for the expertise and responsiveness of their support team. Many G2 reviewers mention that the setup was easier than expected, with the ZenQMS staff being highly knowledgeable and proactive in guiding users through the integration. This kind of customer support seems to be a key factor in users feeling confident about the system and its implementation in their organizations.
I’ve encountered frequent praise for ZenQMS's training management capability. Users frequently highlight how easy it is to track and manage employee training, which has helped them stay on top of training requirements. I’ve seen multiple G2 users express satisfaction with how this feature improves the organization of training efforts and ensures that all necessary training is properly managed.
Additionally, the change control module has garnered positive feedback for its flexibility and effectiveness. G2 users often mention how customizable this module is, allowing them to tailor tasks to meet their specific needs. This customization is seen as a big plus, as it ensures that change control processes stay aligned with the organization’s standards. Similarly, the Issue module receives praise for keeping teams organized and on top of tracking and managing issues, which many users find valuable for maintaining transparency and avoiding overlooked details.

According to G2 user reviews, ZenQMS is recognized for its compliance-focused workflows, though some users find navigation around change controls and issues less intuitive. A more streamlined layout has been suggested to make these processes easier to manage.
Another theme in G2 feedback relates to document management. Reviewers note the lack of features like appendix numbering and automatic Key-ID integration in headers, which would help organize large document sets and reduce manual errors. A few users also mention that an equipment module could strengthen the platform for operations that rely heavily on asset tracking.
Overall, G2 sentiment reflects ZenQMS as a reliable compliance solution, with opportunities to refine workflow navigation and expand functionality for documents and equipment management.
What I like about ZenQMS:
- I’ve seen consistent praise from G2 users for ZenQMS's ease of use, with many highlighting the intuitive platform and quick onboarding.
- G2 users frequently mention that the customer support team is highly knowledgeable, responsive, and makes the implementation process smoother than expected.
What G2 users like about ZenQMS:
"My favorite part of ZenQMS is the flexibility it offers through its modules, allowing us to capture many different processes. It's very user-friendly and has helped us integrate several paper-based processes, making work across multiple locations much more efficient for our small Quality Team. Having used multiple QMS platforms in my career, ZenQMS is by far one of my favorites, and the amazing customer support only strengthens my trust in the system."
- ZenQMS Review, Verified User in Research
What I dislike about ZenQMS:
- I’ve noticed that some G2 users find the Change Control and Issues workflows confusing, with suggestions for a more streamlined layout and better navigation.
- I’ve read feedback mentioning that the lack of the ability to add appendix numbers or integrate Key-ID numbers into document headers is seen as a significant limitation for users handling large documents.
What G2 users dislike about ZenQMS:
"My only complaint about the system currently is that I wish there were a way to arrange the assignments in a specific order, allowing users to follow the list in the order we want them to. I also wish there were a way to place tests and associated training modules together in an individual's dossier, so they don’t have to search for the associated documents."
- ZenQMS Review, Michelle W.

Best Quality Management Software: Frequently Asked Questions (FAQs)
Which QMS software has the highest customer reviews?
According to G2 user feedback, MasterControl, ETQ Reliance, and Greenlight Guru consistently receive top reviews. They are praised for compliance strength, automation, and their ability to handle regulated industries effectively.
What is the most affordable QMS software for startups?
Qualio and SafetyCulture are considered the most affordable options for smaller teams. SafetyCulture even offers a free plan, while Qualio is tailored for growing life sciences startups seeking compliance without heavy costs.
What are the top-rated QMS apps for service businesses?
SafetyCulture is highly rated for service industries that rely on inspections and audits. Its mobile-first design helps teams run checklists, manage safety workflows, and generate reports quickly.
Which is the most user-friendly QMS app for business process management?
ZenQMS stands out as a user-friendly platform. It’s designed for compliance-heavy businesses and offers intuitive workflows for audits, training, and document control without steep learning curves.
What is the most popular QMS service for enhancing software quality?
Ideagen Quality Management is a strong choice for software and service companies. Its focus on risk management, audit trails, and compliance makes it ideal for enhancing overall software quality processes.
What are the leading QMS solutions for the software industry?
ETQ Reliance and QT9 QMS are leading picks for software-focused organizations. They provide automation, analytics, CAPA tracking, and modular workflows to maintain compliance and improve efficiency.
What is the best quality management software for tech companies?
Greenlight Guru and AmpleLogic are popular for tech and manufacturing-focused firms. Greenlight integrates product lifecycle with QMS, while AmpleLogic provides customizable controls for operational efficiency.
What are the best quality management systems for small software firms?
Qualio and QT9 QMS are well-suited for small firms. They are lightweight, cloud-based, and modular, offering CAPA, document control, and audit management without requiring large enterprise budgets.
What is the most reliable quality management service in SaaS?
ETQ Reliance and MasterControl are widely recognized as reliable SaaS-based QMS platforms. They provide flexible automation, compliance assurance, and strong analytics for enterprise-grade requirements.
Who are the best QMS providers for ensuring compliance in tech?
MasterControl, Greenlight Guru, and ZenQMS are often highlighted for their compliance strengths. Each focuses on regulated industries like life sciences, pharma, and medical devices, ensuring strict adherence to standards.
Because good enough isn’t good enough
Poor quality impacts your bottom line and can permanently damage your company's reputation. One product recall, failed audit, or compliance violation can erode customer trust faster than it was built.
Each QMS platform I evaluated offers unique features tailored to different needs, whether you’re focused on preventing defects, streamlining audits, or managing corrective actions.
With so many strong options, there's no reason to leave your quality and reputation to chance. I hope my insights help you find your organization's best management software.
Discover the best vendor management tools to strengthen supplier quality and compliance.
.png?width=400&height=150&name=Untitled%20design%20(56).png)











.png)





.png)