
As a civil engineering student, I loved environmental sciences. We spent a lot of time outdoors, and when we weren’t, we were in the lab conducting interesting tests, mostly on water and soil samples.During one visit to a testing lab, I wondered how every sample was handled so efficiently — how it was sent to the right testing stations, kept safe from contamination, and tracked for accurate results. Curious, I asked my professor, who introduced me to the best laboratory information management system (LIMS) software. A week later, I was using one of these tools to manage soil samples for my entire batch!
That experience showed me how the best LIMS software makes lab work much easier. It helps track samples, keeps data secure, and ensures everything runs smoothly. Over time, I’ve learned what labs need most from these tools, like accuracy, efficiency, and seamless integration with lab equipment.
Now, as a writer who also reviews software on G2, I've combined my own experience and analyzed user reviews on G2 to create this list of the best LIMS software. Read on to find the right one for your lab. Let’s get started!
Thermo Scientific SampleManager: Best for complex labs needing automation
For high-throughput environments that require instrument connectivity, scheduling, and advanced workflow orchestration.
* These LIMS software are top-rated in their category, according to G2's Fall 2025 Grid Report. Pricing for these products is available upon request, except for those I’ve already included the details.
Did you know? The global laboratory information management system (LIMS) market was valued at USD 2.44 billion in 2024 and is expected to reach USD 3.56 billion by 2030, expanding at a compound annual growth rate (CAGR) of 6.22% between 2025 and 2030.
LIMS software helped me efficiently manage and track laboratory samples, ensuring accurate data collection and analysis.
The best LIMS software went beyond traditional record-keeping, automating workflows, minimizing human error, and integrating seamlessly with lab equipment. It became an indispensable tool in my daily work by streamlining lab processes and making research more manageable.
When I first started working in a lab, managing samples and data felt overwhelming. I spent hours tracking down information, sorting through paper records, and ensuring everything was in the right place. Once I started using LIMS software, sample management became far easier and faster. It helped me stay organized, ensured accuracy and freed up time for more critical tasks like data analysis. These tools not only made my work more efficient but also improved the overall quality of my research.
In this article, I’ll share my personal picks for the best LIMS software in 2025. I’ve evaluated each tool and will highlight what made them stand out and how they helped me tackle common lab challenges.
I evaluated the best LIMS software extensively to manage and track laboratory samples, automate lab workflows, and assess its efficiency in ensuring accurate data collection and analysis.
To enhance my understanding, I also spoke with laboratory professionals to learn about their needs and challenges in sample management. I used AI to analyze user feedback and reviews on G2 and G2’s Grid Reports to gain further insights into each tool’s features, ease of use, and overall value.
By combining hands-on evaluations with expert feedback and user reviews, I’ve compiled a list of the best LIMS software to help you select the right one for your lab’s needs.
When evaluating various LIMS solutions, I focused on a few key factors to evaluate how well they address the complex needs of modern laboratories. Here's a breakdown of the aspects I paid attention to when selecting the best LIMS software:
The list below contains genuine user reviews from our best LIMS software category page. To qualify for inclusion in the category, a product must:
This data was pulled from G2 in 2025. Some reviews have been edited for clarity.
Based on my review of G2 user feedback, STARLIMS stands out as a comprehensive laboratory information management system (LIMS) that supports the full spectrum of lab operations. Many reviewers describe it not just as a tool, but as a complete ecosystem that integrates various workflows across a lab's lifecycle.
One feature that I see getting a lot of praise is sample lifecycle management. From what I’ve read, users value how STARLIMS tracks each step, from collection and processing to storage and archival, with precision and clarity. This is especially important in regulated environments, where accuracy and traceability are non-negotiable.
Looking at broader review trends, I can see recurring appreciation for the platform’s scalability. According to G2 users, STARLIMS adapts well to different lab sizes and needs, whether you’re running a small research facility or a high-throughput testing operation. The ability to scale without major system overhauls is often cited as a key advantage for long-term growth.
Across multiple reviews, I’ve noticed users highlight the platform’s ability to bridge research and quality control processes. This transition from development to manufacturing workflows is seen as a major benefit, particularly in pharma and biotech sectors where reducing time-to-market can have a significant impact.
Something G2 reviewers seem to really appreciate is the reporting and analytics capability. Users consistently mention that STARLIMS goes beyond basic reporting by offering deep insights that support informed decision-making. Many also commend its ability to automate compliance documentation and align with FDA and ISO standards, helping streamline audit processes.

The SDMS is highly precise, which helps maintain data accuracy. Some G2 reviewers mention that this precision can make it overly sensitive to formatting discrepancies in instrument files, leading to longer troubleshooting times. Many teams streamline workflows by standardizing file templates and validation checks to minimize friction.
Going digital with ELN is a major advantage for documentation. However, G2 users note that issue resolution within the ELN can be slow during critical moments, occasionally disrupting productivity. Teams that build internal SOPs and maintain clear escalation paths generally see smoother performance.
Manual entry functions reliably for most workloads. G2 feedback indicates that under heavy data volumes, the results entry module can slow down, creating temporary throughput bottlenecks. Optimizing batch uploads and archiving inactive data typically restores responsiveness.
The platform’s feature breadth is a clear strength. Yet, new users often describe a steep learning curve, particularly in SDMS and ELN modules. G2 reviewers recommend structured onboarding, training programs, and gradual module adoption to unlock the platform’s full potential.
Rated 4.5/5 on G2, G2 reviewers consistently highlight the STARLIMS robustness, data integrity, and compliance-readiness as key benefits. With thoughtful onboarding, standardized workflows, and resource planning, most teams transform initial complexity into long-term operational strength.
"STARLIMS offers robust data management capabilities, enabling centralized storage, quick retrieval, and efficient analysis of experimental data. Its comprehensive audit trail function allows users to trace and record the source, processing steps, and results of samples, ensuring the reliability and traceability of the data."
- STARLIMS Review, Xueyan Z.
"The SSL language is quite limited, offering only basic functionality with very few system features. Additionally, there is a lack of detailed and up-to-date documentation for these functions, making it difficult for users to fully utilize the system."
- STARLIMS Review, Soy H.
Maintain efficiency and accuracy in your lab operations. Explore the best lab inventory management solutions today.
Based on my review of G2 user feedback, QBench consistently earns praise for its fast and straightforward setup process. Many reviewers highlight how the platform is easy to get up and running without lengthy onboarding or complex configurations. That simplicity seems to be a welcome change for labs used to more cumbersome LIMS implementations.
One standout capability, according to users, is workflow automation and order tracking. I frequently see G2 reviewers highlight how the system enables real-time monitoring of orders, samples, and test progress. The no-code automation tools are especially well-received, helping labs eliminate repetitive manual tasks and streamline operations — without needing a developer on hand.
A commonly appreciated element is QBench’s flexibility in sample tracking. From what I’ve seen, users value its support for custom fields, which allows teams to tailor the system to their own workflows. This adaptability is cited as a key reason labs across different industries choose QBench over more rigid alternatives.
Something G2 reviewers seem to really appreciate is the platform’s bulk reporting tools. Many users mention how reusable templates simplify the process of generating high-volume reports. This appears to be a huge time-saver for labs with fast turnaround requirements, especially when accuracy and consistency are critical.

QBench’s quick-find and dropdown suggestions make locating records fast once you know the flow. Some G2 reviewers note that pressing Enter after typing a sample number can redirect unexpectedly, which disrupts new users; most sidestep it by selecting from the suggestions or using saved searches.
Out-of-the-box filters like Today and This Week cover common ranges. G2 users would like added options (e.g., Tomorrow) and flag occasional late-night date rollovers that can misplace entries; labs typically standardize shift cutoffs and use custom views to keep timelines clear. Spreadsheet-friendly teams adapt quickly to QBench’s exports and tabular edits. For users without Excel experience, G2 feedback mentions a short learning curve and occasional workarounds for niche workflows; starter templates and brief enablement sessions usually close the gap.
With an overall rating of 4.5/5, G2 reviewers consistently describe QBench as powerful and dependable after initial configuration.
"We've been using QBench for about 8 years, and it has proven to be a modern, modular, and highly customizable LIMS. It offers our laboratory the flexibility to adapt to the ever-changing requirements set by regulatory bodies and our customers. Its robust APIs, responsive local customer service team, and reasonable, scalable cost structure make it a great choice for our needs."
- QBench Review, Zachary E.
"There is a learning curve, and prior experience with Excel or VBA is very helpful for developing the mindset needed to fully utilize the software's strengths. If your processes are already well-documented, the software can assist in implementing them, which helps reduce the workload for administrators."
- QBench Review, Aaron R.
Thermo Scientific SampleManager is frequently described by G2 reviewers as a robust laboratory information management system (LIMS) that offers powerful data analytics through pre-configured dashboards. These dashboards are interactive and present key lab metrics, such as resource availability, inventory status, and performance indicators, in a user-friendly way. From what I’ve read, users consistently praise how easy it is to extract insights without needing to dig too deeply into the data.
Another commonly appreciated element is the deployment flexibility. According to feedback I gathered from G2 users, the option to deploy either on-premises or in the cloud gives labs more control over infrastructure decisions. Many find this useful for balancing IT costs and system security, with cloud deployment offering added benefits like disaster recovery and reduced hardware dependency.
One standout capability, based on several reviews, is the Laboratory Execution System (LES). G2 users report that LES provides clear, step-by-step process guidance that helps ensure SOP compliance. I’ve also seen reviewers mention how well the system manages training records, allowing labs to assign and track training by role and enforce access controls to maintain compliance before tests or equipment usage.
I frequently see G2 reviewers highlight SampleManager’s traceability features, especially how it captures actions and process history. Reviewers say this visibility makes audits and process reviews much easier. In addition, the contract lab portal receives positive attention for simplifying quote, sample, label, and invoice management, all within a secure interface.
From what I’ve seen, integration with the Chromeleon Chromatography Data System (CDS) is another highly valued feature. Users report that it makes handling chromatography and mass spectrometry data smooth, centralized, and secure, which is especially useful for labs managing complex analytical workflows.

Analyzer links can deliver seamless data flow once configured. Some G2 reviewers report that establishing analyzer-to-computer connections may require programming know-how, which is tough without developer support; labs typically smooth this by using vendor connection kits, standard HL7/ASTM profiles, and a short, assisted onboarding. Normal operations are stable for most teams. A few G2 users note that rare server issues can pause sample processing entirely; high-uptime labs often add failover, scheduled maintenance windows, and monitoring alerts to keep workflows moving.
Imports handle standard cases reliably. Multiple G2 reviews mention rigidity with formats like .xls and .cdx, creating error-prone steps versus drag-and-drop tools; template-based CSVs and guided import wizards usually reduce friction.
The platform is highly configurable and supports compliant processes. G2 users say specialized workflows can involve trial-and-error early on; starter SOPs, sandbox testing, and vendor solution-architect sessions help teams reach “steady state” faster.
The core web experience covers most lab tasks. G2 feedback frequently calls out limited mobile access, which constrains real-time monitoring; many labs address this with secure VPN + browser access or lightweight mobile dashboards for key KPIs.
With a 4.3/5 rating on G2, reviewers consistently highlight strong process standardization, auditability, and compliance once configurations are in place.
"As the Lab Supervisor, we needed a solution that could grow with us, both now and in the future, and SampleManager has delivered on that promise. We’re a contract lab that tests in-service lubricants, handling numerous clients and various pieces of equipment. When evaluating potential LIMS software, SampleManager stood out and has certainly met our expectations. The biggest benefit so far has been the significant improvement in turnaround time by streamlining the logging and reporting processes. This has been a major win for our team."
- Thermo Scientific SampleManager Review, Craig B.
"The speed is an issue. Despite using AWS within the company, some Windows load very slowly. For example, modifying folders takes around 20 to 30 seconds from clicking to opening. Software should open windows within half a second to ensure it feels fast and usable."
- Thermo Scientific SampleManager Review, Raul P.
Ensure seamless audits and stay compliant — integrate compliance software with your LIMS today!
Labguru ELN LIMS is often recognized by G2 reviewers for its streamlined sample and inventory management. From what I’ve seen, users consistently appreciate how easy it is to track where samples and supplies are stored, with automatic updates to inventory levels in real time. This level of visibility helps teams manage stock efficiently, something that’s especially valuable in labs dealing with high volumes of consumables and samples.
One feature that I see getting a lot of praise is the certification analysis tool. G2 reviewers describe how it allows users to digitally sign off on experiments, witness entries, or reject them based on set standards. This functionality supports better documentation and accountability, which reviewers say is critical for labs with strict quality assurance protocols. The shift away from paper-based records toward digital compliance is something users seem to really value.
According to feedback I gathered from G2, instrument management and orchestration also stand out. Users mention how helpful it is to monitor calibration or set equipment maintenance timelines. This proactive scheduling reportedly reduces downtime and improves lab efficiency, which I can imagine is a major win for lab managers overseeing complex instrument fleets.
Something G2 reviewers seem to really appreciate is the platform’s ability to handle large datasets. I’ve read that uploading and exporting sizable research files is smooth, and system performance doesn’t degrade even when working with heavy data loads. This is especially useful in data-intensive environments, where speed and data integrity are non-negotiable.

The mobile app is handy for quick checks and basic actions. Some G2 reviewers note it lacks desktop parity, tasks like report review or editing experimental workflows aren’t available on mobile, which can limit fully on-the-go use. Teams often reserve complex edits for desktop and use mobile for status, approvals, and field notes.
Core location tracking works for everyday moves. Across G2 reviews, labs needing ultra-granular mapping (e.g., multi-compartment ULT freezers) want finer coordinates; many address this with standardized naming schemas (rack/shelf/box/slot) and barcode labels to add precision.
File uploads centralize documentation. G2 users mention that documents aren’t always tied to specific events (service, calibration), making audits slower; linking via SOPs, consistent naming conventions, or periodic record reconciliations improves traceability until native event-level links expand. Inventory tracking covers routine stock visibility. Several G2 reviewers would like automatic alerts for nearing expiration to prevent waste and delays; labs commonly add scheduled reports, dashboard flags, or simple reminder rules as a stopgap.
Despite these gaps, Labguru ELN LIMS is rated 4.6/5 on G2 and is widely regarded as a capable platform. Based on G2 feedback, its strengths lie in compliance tracking, equipment scheduling, and managing research data at scale. It’s clear why this tool appeals to modern labs that prioritize both operational efficiency and regulatory rigor.
"LabGuru makes it very easy to prepare protocols for experiments and track progress during a procedure. It allows different people to swap in and out, and it’s clear who has performed each step, ensuring accountability and smooth workflow."
- Labguru ELN LIMS Review, Linda S.
"LabGuru offers so many features that it can be overwhelming to navigate. I know I’m not using all of its capabilities simply because there’s too much information to absorb at once."
- Labguru ELN LIMS Review, Erica W.
Genemod is a cloud-based LIMS platform built for biotech R&D teams. From what I’ve gathered while analyzing G2 reviews, its cloud accessibility is frequently highlighted as a major strength, especially for distributed teams. I’ve seen users appreciate being able to log in from anywhere, making it easier to collaborate remotely and manage projects across lab locations.
The electronic lab notebook (ELN) receives consistent praise in G2 feedback. One standout capability, according to users, is how simple it is to organize experiments, projects, and folders. There’s a lot of love for the real-time collaboration it enables — users mention that changes sync instantly, streamlining teamwork. A commonly appreciated element is the ability to store and share protocols in a centralized, secure location, cutting down on time spent searching for scattered files.
I frequently see G2 reviewers highlight Genemod’s data integrity features. Built-in audit trails and version control tools are considered crucial by users working in regulated environments. Based on my review of user comments, many value how clearly the platform tracks changes, identifying who updated what and when, helping labs stay compliant and accurate.
Customizability also stands out in the feedback I reviewed. Users often mention how easy it is to tweak the platform to fit their specific lab processes. That flexibility appears to be a key reason many teams stick with Genemod after adoption.
According to feedback I gathered from G2 users, security features are another area where Genemod performs well. Reviewers regularly mention strict access controls and strong data protection protocols. There’s clear appreciation for how the platform handles confidentiality and compliance.
Across multiple reviews, I’ve noticed users mention particular satisfaction with inventory management. The interface is described as intuitive, with features that make it easy to track supplies, monitor stock levels, and keep tabs on expiration dates — something many say improves lab efficiency and reduces waste.

Printing on-demand labels is reliable once items exist in the system. Some G2 reviewers note that pre-printing labels for upcoming samples isn’t supported, which slows high-throughput setups. Labs often work around this by creating placeholder records or integrating external label tools for advance runs.
The dashboard provides clear visibility into key lab metrics. However, G2 users mention limited flexibility in rearranging or prioritizing tiles, which constrains personalization. Many teams handle this by using saved views or shared dashboards tailored to specific roles.
Standard cryo box layouts meet most tracking needs. G2 reviewers working with LN2 freezers or custom layouts would like broader format support; naming conventions and detailed hierarchy structures help bridge the gap until those options expand.
With a 4.8/5 rating on G2, reviewers consistently highlight Genemod’s intuitive interface, collaboration features, and streamlined molecular biology workflows. With minor UI refinements and expanded report and storage options, it continues to deliver strong value for modern research teams.
"In our busy lab, we often struggled to keep samples and reagents organized, but using Genemod has completely transformed our workflow. The user-friendly freezer inventory feature makes it easy to track exactly what’s stored and where, while the repository provides a clear, at-a-glance overview of our entire inventory. This helps us stay on top of what we have and what needs restocking, making lab management much easier and more efficient."
- Genemod Review, Robert D.
"Some features, like the sequence workspace, are still being developed. Additionally, there are small issues, such as when exporting freezers from Genemod to CSV files, the box names are replaced with internal box IDs, which requires manual changes. It can also be challenging to quickly view box information because, at the moment, only compound names are displayed in each box cell. As a result, details like concentration, mass, and other important information can only be seen by clicking on individual items or adding them to the compound name."
- Genemod Review, Christopher O.
By integrating medical lab software with your LIMS system, you can fine-tune your operations and set up seamless workflows.
In reviewing LabWare LIMS, one of the first things I noticed from G2 reviewers is how much customization the platform allows. Users often praise the system’s flexibility, especially in settings where lab processes differ significantly. The modular architecture is another aspect that’s frequently highlighted, as it lets organizations tailor features to match their specific workflows.
Based on user feedback, the cloud-based deployment model is seen as a major advantage. It simplifies the setup process and reduces the time needed to get started. A feature that G2 reviewers consistently call out is the visual workflow builder, which helps make system configuration clearer and more intuitive. According to multiple reviews, labs can go live in under 30 days using LabWare’s pre-configured SaaS offering — something many see as a welcome alternative to long deployment cycles.
One standout capability, according to users, is the internal change management system. Reviewers appreciate how this tool tracks updates and changes in an auditable way, which is especially important for regulatory compliance. The flexibility to customize this system based on lab needs is a benefit that comes up often in reviews.
There’s a lot of love for the way this tool handles scanner and tablet integrations. Reviewers mention that collecting and processing data in real time is seamless, with minimal disruption to ongoing tasks. These features seem to help labs operate more efficiently and improve daily productivity.
Another frequently praised aspect is the stability study management functionality. G2 users note that it supports everything from inventory and storage location tracking to sample chain of custody, results entry, and reporting. This end-to-end management appears to be a big time-saver for labs running complex stability studies.

Granular facility/plant settings enable precise control across sites. Some G2 reviewers note that adjusting these during initial setup can be time-consuming and adds complexity; teams typically streamline by starting with a baseline template, then layering site-specific deltas.
Prebuilt templates accelerate standardization. From G2 reviews, default analyses/subroutines in imported templates don’t always match custom workflows, creating clutter; most labs curate a “golden” template set and retire unused steps to keep navigation clean.
The platform’s modules cover a wide range of use cases. G2 users mention inconsistent visuals, some modules feel modern while others look dated, which can slow switching; role-based dashboards and pinned favorites help maintain flow until UI parity advances. Depth and flexibility are core strengths. Multiple G2 reviewers say formal training is essential to unlock value; teams that schedule vendor-led onboarding, SOPs, and sandbox practice report faster time-to-proficiency and smoother configuration.
Overall, based on the G2 feedback I’ve gathered, LabWare LIMS is a strong option for labs that need a highly customizable and scalable solution. Rated a strong 4.5/5 on G2, teams should be prepared for a potentially steep onboarding process and a need for structured training to make the most of what the platform offers.
"Labware LIMS offers predefined templates that provide a structured framework for your database and LIMS system. It also allows you to customize the interface and processing templates to meet your specific needs."
- LabWare LIMS Review, Keegan W.
"The user interface is not intuitive, and the visual workflow solution only offers a basic enhancement, ultimately leading users back to outdated data entry and admin screens that haven’t been updated in 20 years. Additionally, with minimal out-of-the-box functionality, the setup process requires more time and effort for tedious configurations compared to other LIMS products."
- LabWare LIMS Review, Gerry Y.
While evaluating CloudLIMS, I frequently see G2 reviewers highlight its instrument integration capabilities. Users mention that the system handles data transfers from a wide variety of instruments effortlessly using CSV/XLS files. From what I’ve read, CloudLIMS automatically reads these files and maps the data to the right fields, which helps eliminate manual entry and ensures consistency in lab operations.
Another commonly appreciated element is the platform’s reporting and certificate of analysis (CoA) customization. According to feedback I gathered from G2 users, the ability to design multiple report templates at no extra cost is a huge benefit. Reviewers seem to really appreciate the flexibility to meet both regulatory requirements and client preferences — especially for labs working with diverse stakeholders.
The functionality around legacy data migration stands out in reviews. Many users point out how smooth and secure the process is, even when transferring data from older systems. I’ve come across multiple mentions of successful migrations without data loss or corruption, which is reassuring for labs switching platforms.
A feature that gets frequent praise is data security and backup. Based on my review of G2 user feedback, CloudLIMS runs regular automated backups and stores them across geographically distributed servers. Users feel confident that even in the event of a disaster, their data will remain safe and recoverable.
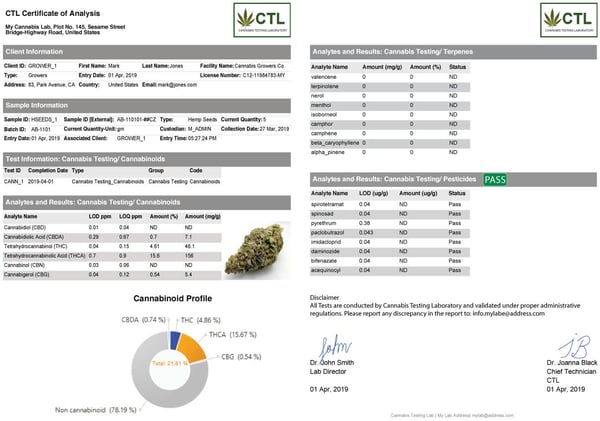
Recent updates have improved dashboarding, and standard widgets cover common needs. Some G2 reviewers still find setup in older versions unintuitive for non-technical users; teams usually standardize a few starter dashboards and clone them per role to speed adoption.
Core workflows are easy to reach once familiar. G2 users note that certain windows use column layouts that feel clunky and slow, and many request richer sample status states (e.g., “picked up,” “received”) for clearer handoffs; saved views and custom fields help bridge visibility gaps. Day-to-day responsiveness is generally solid. A few G2 reviews mention occasional lags during peak usage; scheduling heavy jobs off-hours, archiving older records, and using filtered lists typically keep throughput smooth in time-sensitive labs.
Overall, CloudLIMS holds a strong 4.5/5 rating on G2 and reviewers consistently highlight its quick, cloud-native deployment, real-time sample tracking, and compliance-friendly records. With a few governance patterns (starter dashboards, saved views, guided setup), most labs get fast time-to-value and a platform that scales with their workflows.
"The CoA template was customized to meet our needs, including all essential client and sample details, along with a table displaying analytes and their observed values. Each table cell was designed to capture values based on specific test types. The CloudLIMS team collaborated closely with us to ensure the template matched our expectations. They automated calculations and applied built-in logic and complex algorithms to make this happen."
- CloudLIMS Review, Ronel C.
"The system’s session timeout is quite restrictive, as the platform prompts session expiration after a short period. It would be helpful if there were an option to extend the session duration, giving users more flexibility to complete tasks without the concern of losing their progress."
- CloudLIMS Review, Aryan K.
Have more questions? Find the answers below.
According to G2 Data, STARLIMS and LabWare LIMS lead the pack for regulated sectors. G2 reviewers praise STARLIMS for its end-to-end data management, audit trails, and compliance with ISO and GxP standards, while LabWare earns top marks for scalability across multi-site and multi-industry deployments.
For smaller teams, CloudLIMS and QBench are top choices. CloudLIMS offers an affordable cloud-based model with real-time sample tracking and easy integration, while QBench stands out for its low setup overhead, flexibility, and simple pricing.
Enterprise reviewers on G2 most often recommend LabWare LIMS and Thermo Scientific SampleManager. Both platforms support complex workflows, instrument connectivity, and high-throughput automation ideal for global lab operations.
Labguru ELN LIMS and Genemod perform best for research environments. Labguru combines LIMS and electronic lab notebook (ELN) features to streamline documentation and inventory, while Genemod appeals to molecular biology and genomics teams for its intuitive, modern interface.
CloudLIMS tops this category for offering a fully browser-based platform with no local installation. Reviewers highlight its scalability, real-time data access, and seamless integration with analytical instruments and third-party tools.
Thermo Scientific SampleManager is widely regarded for advanced automation, scheduling, and robotic instrument control. G2 reviewers note its ability to handle complex lab operations with minimal manual input.
STARLIMS and LabWare LIMS are designed with compliance in mind, offering audit trails, version control, and secure electronic signatures to meet FDA 21 CFR Part 11 and ISO 17025 requirements.
QBench and Genemod rank highly for ease of use. Their clean interfaces and straightforward setup make them ideal for labs implementing LIMS software for the first time.
Labguru ELN LIMS excels in educational and collaborative environments thanks to its combined ELN/LIMS features, while CloudLIMS offers flexible plans for universities and consortium-based research.
According to G2 reviewers, modern LIMS platforms help labs centralize sample tracking, automate data capture, and maintain compliance. This results in better operational efficiency, reduced manual error, and faster audit readiness across regulated and research-driven industries.
After thoroughly reviewing the top LIMS software available, I can confidently say that the right solution for you really depends on your lab's unique needs. Throughout this journey, I’ve explored the features, benefits, and potential limitations of each option, and I’ve come to realize that no one-size-fits-all approach works here.
Some software excels in user-friendliness, making it easy for teams to adopt with minimal training, while others shine in complex reporting capabilities or integration with other systems.
For me, the key takeaway is this: the best LIMS software simplifies your workflows, saves you time, and gives you confidence in the data you’re managing. It’s like picking the right lab partner — you need someone who knows their stuff, doesn’t create chaos, and can help you tackle any challenge that comes your way.
Ready to elevate your lab's performance? Start exploring the power of quality management systems (QMS) today.
Devyani Mehta is a content marketing specialist at G2. She has worked with several SaaS startups in India, which has helped her gain diverse industry experience. At G2, she shares her insights on complex cybersecurity concepts like web application firewalls, RASP, and SSPM. Outside work, she enjoys traveling, cafe hopping, and volunteering in the education sector. Connect with her on LinkedIn.
No matter your industry, demonstrating your lab's reliability and ability to comply with...
 by Nicholas Evans
by Nicholas Evans
Before I became an SEO content specialist, I studied civil engineering.
.png) by Devyani Mehta
by Devyani Mehta
In the world of laboratories, precision and efficiency are absolute essentials. Yet, inventory...
 by Shonali Paul
by Shonali Paul
No matter your industry, demonstrating your lab's reliability and ability to comply with...
 by Nicholas Evans
by Nicholas Evans
Before I became an SEO content specialist, I studied civil engineering.
.png) by Devyani Mehta
by Devyani Mehta


